Abstract
Background: The thrombopoietin (TPO) receptor c-mpl is expressed on the surface of megakaryocytes and platelets. In addition to its effect on increasing megakaryocyte growth and maturation, TPO also lowers the threshold for platelet activation in vivo and in vitro . TPO receptor agonists (TPO-RA) may, perhaps to differing extents, also lead to platelet activation. Indeed, in a recent study of patients with chronic liver disease (CLD), the TPO-RA eltrombopag reduced the need for platelet transfusions, but was associated with an increased incidence of thrombosis. Therefore, the goal of this study was to evaluate the effect of avatrombopag, a novel TPO-RA, on in vivo platelet activation and in vitro platelet reactivity in response to agonists in patients with CLD.
Methods: CLD patients participating in the Phase 3 E5501-G000-310 and -311 studies (Randomized, Global, Double-blind, Placebo-controlled, Parallel-group Studies to Evaluate the Efficacy and Safety of Once-daily Oral Avatrombopag for the Treatment of Adults with Thrombocytopenia Associated with Liver Disease Prior to an Elective Procedure) could participate in a substudy to evaluate the effects of avatrombopag on platelet function. IRB-approved informed consent was obtained prior to sample collection. Patients (n = 30) were randomized to treatment on Days 1 - 5 with placebo or avatrombopag: 60 mg daily in patients with platelet counts <40 x 109/L, and 40 mg daily in patients with platelet counts 40 to ≤50 x 109/L. Samples were collected at Baseline (Day 1, pre-drug) and after treatment (Day 4 and Day 10). No patient received a platelet transfusion within the 10 days prior to these blood draws.
Platelet activation was evaluated by whole blood flow cytometry (which, unlike other methods, is accurate in samples with low platelet counts) with and without in vitro stimulation with adenosine diphosphate (ADP) and thrombin receptor activating peptide (TRAP). Endpoints included platelet surface activated glycoprotein (GP) IIb-IIIa (as reported by the activation-dependent monoclonal antibody PAC1), platelet surface P-selectin, platelet surface GPIb, platelet phosphatidylserine expression and generation of platelet-derived microparticles. Citrate-anticoagulated whole blood was incubated (15 min, room temperature) with an antibody cocktail containing fluorescein isothiocyanate (FITC)-conjugated PAC1, phycoerythrin (PE)-conjugated anti-P-selectin, and PE-Cy5-conjugated anti-CD42b (GPIb) with or without ADP 0.5 µM or 20 µM or TRAP 1.5 µM or 20 µM. Samples were fixed with 1% formaldehyde, then shipped cold together with the remaining blood sample (for analysis of phosphatidylserine expression and microparticles) to the Center for Platelet Research Studies for analysis. Phosphatidylserine expression on platelets was determined by staining with FITC-conjugated annexin V. Platelet-derived microparticles were identified by light scattering properties and positive staining for CD41 and CD42b. Changes from Baseline for drug-treated (60 mg and 40 mg avatrombopag cohorts combined, n=20) vs. placebo-treated patients (n=10) were compared using a mixed effects model with p<0.05 being considered significant.
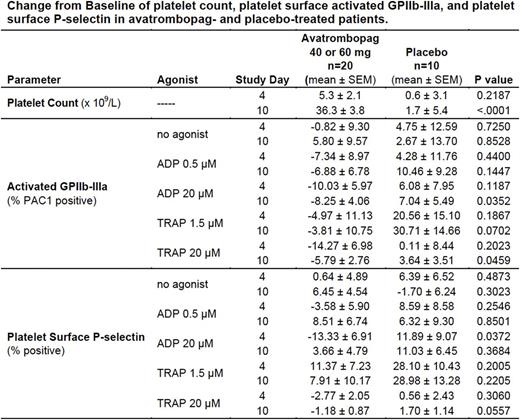
Results: Avatrombopag, 60 mg and 40 mg treatment, but not placebo treatment, resulted in significant increases in platelet counts at Day 10 (Table). Circulating activated platelets were not increased in avatrombopag-treated patients compared with placebo-treated patients (Table, "no agonist" data), as measured by platelet surface activated GPIIb-IIIa and platelet surface P-selectin. Platelet reactivity, as measured by platelet surface activated GPIIb-IIIa and platelet surface P-selectin after low and high concentrations of ADP and TRAP, was also not increased in avatrombopag-treated patients compared with placebo-treated patients (Table). Similarly, there was no evidence of increased platelet activation in avatrombopag-treated patients compared with placebo-treated patients as assessed by platelet surface GPIb, platelet phosphatidylserine expression and generation of platelet-derived microparticles.
Conclusions: In this randomized, double-blind, placebo-controlled, parallel-group study of patients with thrombocytopenia due to CLD, treatment with avatrombopag resulted in increased platelet counts, but did not increase either platelet activation in vivo or platelet reactivity in vitro .
Frelinger: Ionis: Research Funding; Sysmex: Research Funding; Pfizer: Research Funding; Ironwood: Research Funding; GLSynthesis: Research Funding; Baxalta: Research Funding. Michelson: Elsevier: Patents & Royalties; Eisai: Research Funding; GLSynthesis: Research Funding; Instrumentation Laboratory: Consultancy; Baxalta: Research Funding; Ionis: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Sysmex: Research Funding; Pfizer: Research Funding; Ironwood: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal